Chemistry 113: A is for...
... ass-kicking?
Because that's what I did in Chemistry 113 this semester! I made a 96.8% overall, good for an A and 0.2 points shy of an A+!
I pulled a 98% on the final exam - courtesy of a 6-point curve, which I delightfully helped set since the aspiring Taco Bell assistant managers and future Wal-Mart greeters that are my classmates AVERAGED a 63.5% on said final. (Not that I have anything against Taco Bell assistant managers or Wal-Mart greeters since they are taxpaying contributors to our society, but that I don't want them removing my appendix. Please re-read that statement: They AVERAGED a 63.5% - it wasn't a mean score. They had to work hard to drag me down.)
So what's next... well, Chem 2, Bio 1, Bio 2, Physics 1, Physics 2 and Organic Chem 1 and 2. And then I get to take the MCAT and apply to med school. Whew!
Right now, I'm sweating out the failure rate of my classmates because without at least a quarter of them going the way of the Colorado pikeminnow I won't be able to take Chemistry 2 as I need to take classes that don't interfere with my gainful employment and taxpaying contributions to society. Due to recent budget cuts, there's only one Chemistry 116 class that fits those criteria (and the Biology and Physics offerings don't conform too well either). Sadly, as a non-degree graduate student, I get to PAY grad-student rates for freshman-level, weed-out classes, and I also get the privilege of registering LAST for said classes. Ergo, I get the dregs... and I get to pay through the nose for it.
That said, even if I don't make it into Chemistry 116 - or freshman-level chemistry 2 for civilians - I wouldn't have traded the experience for the world. Read on to learn what I've been learning...
Lesson 1: Don't take a knife to a gunfight - or, why everyone will want to be my lab partner if I make it into Chemistry 116 next semester.
Behold, our poster presentation - worth 20% of our lab grade.
Our project demonstrates how the gas laws work by using the ideal gas equation to determine the mass of the gas produced by a chemical reaction: PV = nRT (Pressure x Volume = Moles of Gas x Gas Constant (0.08206) x Temperature). Now, my good lab partner Sunburn was skeptical when I brought my camera to the lab to take photos of our little experimental adventure and he was doubly concerned because I said I'd put together the entire poster display after he completed all the problem-solving and math. (Baby Mama Lab Partner wasn't really involved in the equation - she just showed up and read the index card I printed out for her). After I unleashed the awesome powers of scrapbooking domination on the poster, our classmates gasped in awe - I kid you not. In fact, even Sunburn who is a huntin', fishin' good ol' boy said, "Damn, that really is pretty." Even the sorority girls in class recognized the good work and asked me for the name of my paper, embellishment and adhesive source: Shout out to Scrapbooking Etc., in Mesa - locally owned - and yes, the cost of these supplies is tax-deductible as an education-related expense. Woo-hoo!
Here are the classmates who did their poster presentation before us... look closely, you might otherwise miss the tiny poster on the chalkboard eraser-tray. They glued pennies to their poster as an artistic representation of copper. Obviously, I blurred out their faces to hide their shame.
After all six lab teams completed their poster presentations, our Teaching Assistant said we could take our projects home. I told her that I would not be taking ours home as I hoped to see it hanging in the chemistry department's poster presentation "Hall of Fame" - glass display cases lining the hallway leading to the labs an the Learning Resource Center. Here's our competition:
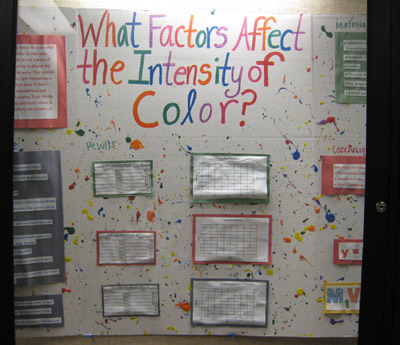
Looking at this example of past poster prowess in the Hall, Pat frowned and said, "Well, at least they tried - they splattered some paint on it to give it some color." I think it looks like someone threw up on it, but that's just me. The next day, our Teaching Assistant sent us the following missive: "Just wanted to tell you that your poster was great and was appreciated in the Department meeting too. That was the best poster among all the classes of General Chemistry. Keep up the good work!"
Not sure if praise in the department meeting will provide an easy entree into the after-work Chemistry 116 section, but don't think I won't use it if sufficient numbers of my classmates fail to fail and become kinesiology majors.
And obviously this is the long way to Lesson 2 of What I Learned in Chemistry 113: Learning is a lifelong sport.
I know that sounds as dorky as all get out (and don't worry, grades have already been posted and I gave my instructor, Dr. Younger Than I, a very nice bottle of wine and hand-written thank you note as a token of my gratitude. I'm in sales - it's what I do.) Still, the sentiment is the same: Not everyone has to be a now-37-year-old pre-med student to fire up the gray matter for a couple laps around the academic track. If I've learned anything about myself this past semester, it's that I need to be challenged, even if it means wearing through a half-dozen No. 2 pencils en route to determining the number of atoms released through the carbon dating of Juan's father to discover his correct age.
Does this mean I'd go back to get an MBA or go to Law School if this whole med school thing doesn't work out? Probably not - the most interesting part of my chemistry pursuit comes from the fact that it is so foreign to me... and it's has so many cool applications. The Graham's Laws of Effusion and Diffusion have explained why Winslow's farts can rip all three of us from the bonds of deep sleep: The methane molecules are relatively small and move through the air more quickly than larger molecules. Depending on whose head is closer to the origin of fart determines who smells it first, but the other poor soul isn't too far behind. I spout this stuff all the time now... Pat can't wait for Biology.