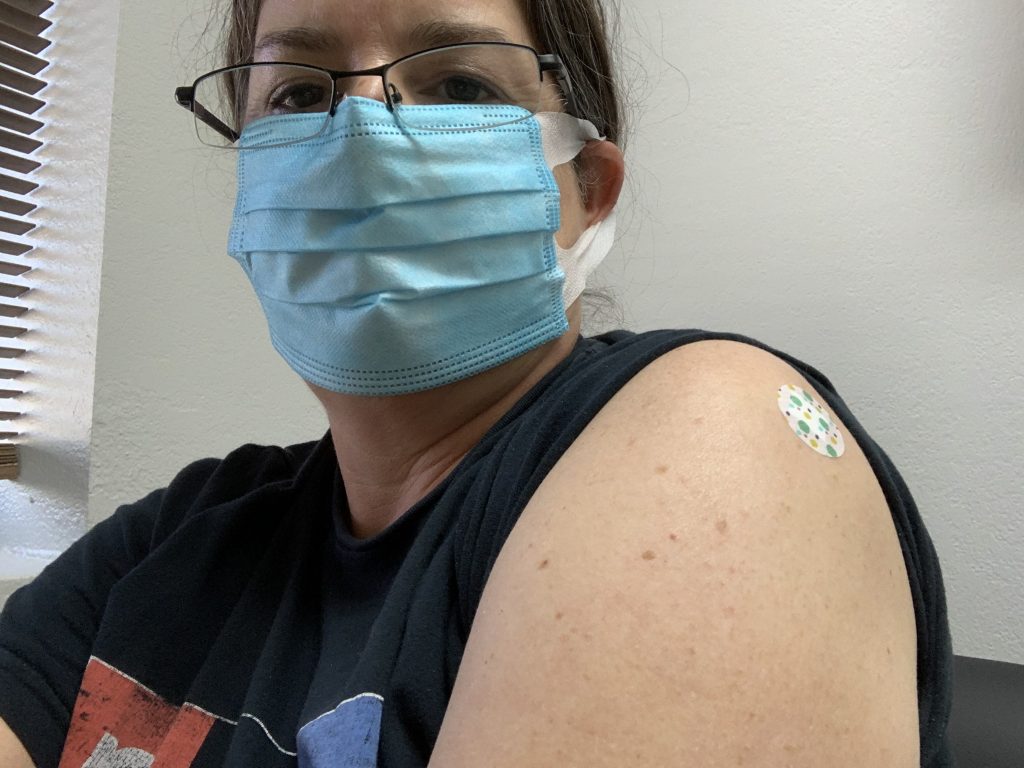
My head hurts.
Is it allergies? Is it stress from the white-knuckle madness of Los Angeles traffic? Is it the two glasses of red wine I drank last night?
Is it a cellular response to a self-inflicted invader? The harbinger of Immunity.
I won’t know for certain for two years.
Yesterday, I enrolled in the AstraZeneca COVID-19 Vaccine Phase III Clinical Trials (AZD-1222), volunteering my blood and guts to fight the greatest threat to humanity since the Cold War: SARS-CoV-2, which has infected 60 million people worldwide since December 2019, killing 1.4 million of them.
As opposed to the sexier mRNA-based vaccines developed by Pfizer / BioNTech and Moderna, the AstraZeneca vaccine is vector-based: It uses a common cold-type virus as a Trojan horse to introduce an altered gene for the coronavirus protein into my cells in hopes of training my immune system to recognize and destroy COVID-19.
Or not.
It’s a double-blind trial, so I will not know if I got THE shot or merely a shot for two years. Until then, I will wallow in hypochondria – or at least for the next 48 hours. The clinical trial nurses advised me to watch out for certain side effects during the first two days:
- Headache
- Fatigue
- Fever or chills
- Muscle or body aches
- Diarrhea or nausea
I will spare you the gurgling details, but my stomach is upset.
Is it a side effect of THE shot? Or merely a symptom of yesterday’s truck stop feast of Lays Limon-flavored chips, Oberto peppered beef jerky, orange-flavored Gatorade Zero, and Australian Licorice (assorted fruit flavors)? Don’t judge.
Enrolling in the trial was a breeze: Sign up online and wait for a call.
AstraZeneca is still recruiting volunteers and especially needs African-American, Latinx, and Native American participants. It’s important because people of color are historically underrepresented in clinical trials – in part because these populations have been victimized by unethical practices, see Henrietta Lacks, Tuskegee Health Benefit Program, et al, and also because lazy researchers didn’t actively recruit and study populations that reflect the diversity of our nation. Most critically, African-Americans, Latinx and Native Americans have been disproportionately affected by COVID-19 – and are more likely to die from it – so volunteering for the vaccine trial can help scientists identify differences that may not be apparent if a bunch of white people like me show up.
My left shoulder hurts. My knees bend audibly. Intermittent pain steals through my joints: Are these the aches they warned me about? The physiological echo of an existential battle inside my cells?
Or is it just a by-product of being a 48-year-old woman who has crashed her mountain bike on more than one occasion?
My day started at the appointed 8 AM in a nondescript doctor’s office in Phoenix. They reviewed the 26-page consent form, which I had read prior to my visit and which detailed my rights as a volunteer: I can withdraw at any time, no questions asked. If I contract COVID, they will funnel me into a different study and manage my care. I will have two shots spaced 29 days apart of either the real deal or a placebo, and 66 percent of volunteers receive the real deal. I will have seven visits to this doctor’s office over the next two years and several phone calls as they monitor my progress.
Sign here and here and here. Initial there and there and there.
Then the delightful Matt did a blood draw, from which they’ll extract my genetic material for study.
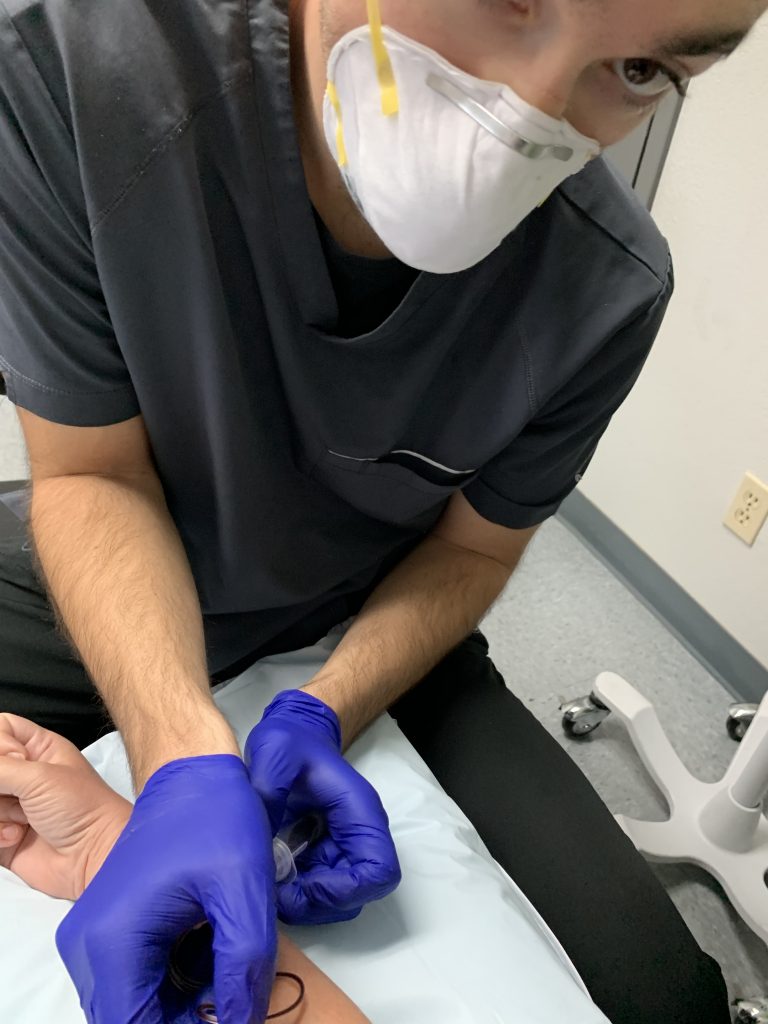
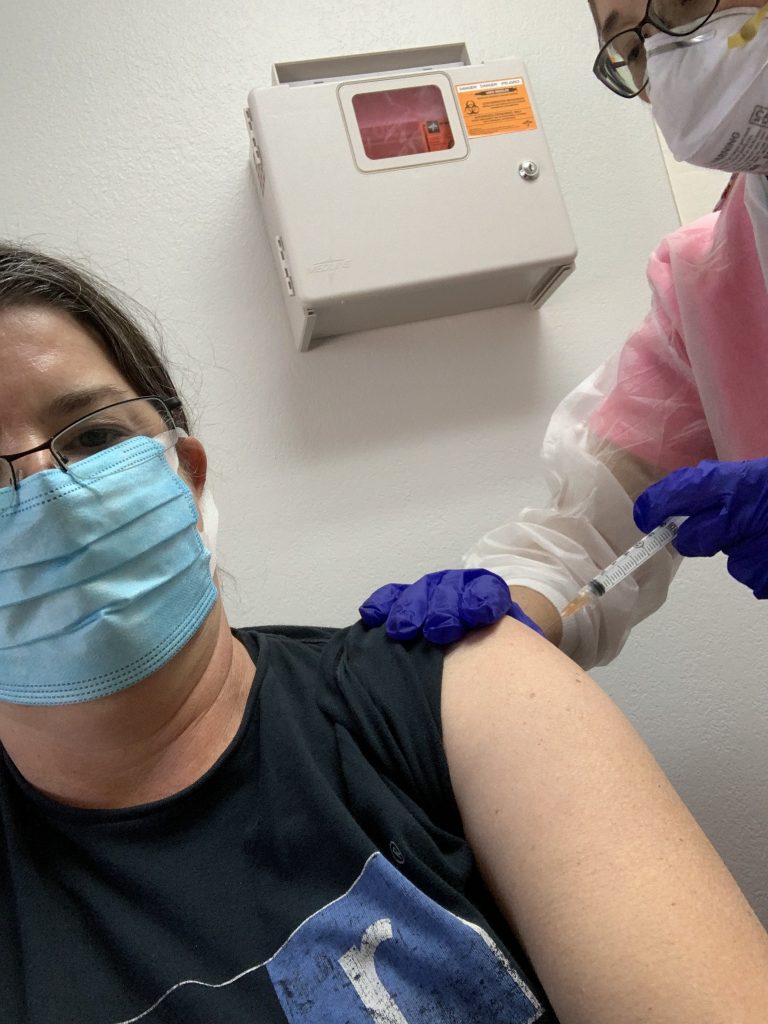
Then I got a physical exam from the doctor and walked down the hall to meet my destiny.

The nurses walked me through the consents again and reminded me of the aforementioned symptoms to monitor for the next 48 hours. I went to bed at 8:30 last night and slept soundly until the splitting headache awakened me at 4:27. I got winded climbing the stairs then fell asleep in the hammock this afternoon. Are these the telltale signs of vaccine-induced fatigue? Or just symptoms of middle age?
I won’t know for another two years, but even if I learn that I got a shoulder-full of saline solution, at least I’ll know I did my part.
You are an inspiration. Thank you for your adventurous spirit.
You’re the best, Stacy. Thanks.
Thanks, friend! It’s not too late to sign up!
How are you feeling?
Feeling normal. The first 24 hours was weird – I really couldn’t tell if what I was feeling was “normal middle age” OR if it was a magical immune response. But now I’m back to normal, and by that I mean, creaky, achy and out of shape!
Very interesting Stacy! So as you won’t get the vaccine when it officially rolls out, were you advised to still wear a mask and take precautions until we reach sufficient immunity (however that is assessed) in 2021?
There is a very lively debate ongoing right now about whether it is ethical to withhold potential life-saving vaccines from people who have volunteered for the trials. Right now, we don’t know if I am placebo or the real thing, but these discussions are underway. In all likelihood, they will decide that trials volunteers should get “the real thing” and we will be notified thusly. Until then, they warned us sternly to follow all precautions – masks, handwashing, social distancing. Currently we are in California under a stay-at-home order so it’s not hard to comply with that.